
It is a tale very commonly told that Lead Acid batteries are a “cheaper” solution than Lithium batteries for off-grid and solar storage. As of 2019 that is patently false, and the purpose of this article is to explain how this remarkable fact comes to be.[1]This argument is true for LiFePO4. For LiMnCo batteries such as “Tesla” modules, this economic argument does not hold water as LiMnCo chemistry cells have a cycle life of 400 cycles or … Continue reading
1. Usable capacity
Lead Acid batteries are infamously printed with very high capacity ratings on their cartons. It’s not uncommon to see “200 Ah” 6-volt batteries available for $90 at stores in the United States, but like many things in modern commerce, there is some important fine print to consider.
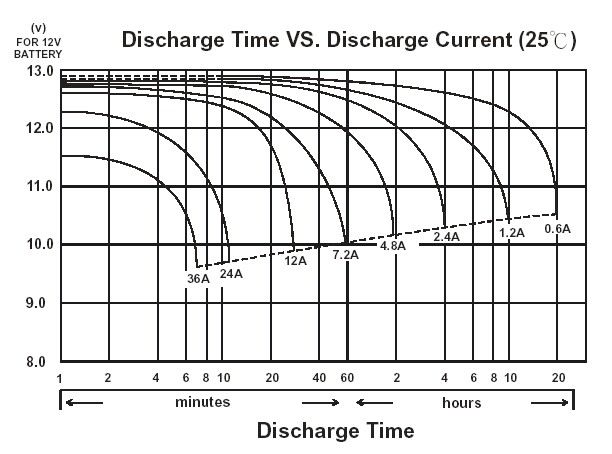
These capacities are universally advertised under “light-load” conditions –usually, a few ampere’s worth of load or less. In this case the battery can provide about 1 ampere for 200 hours which makes for an accurate advertisement.
Under realistic load conditions however, say, 50 amps, there is a different story altogether. Lead Acid batteries are very terrible at maintaining their capacity under realistic loads, and at a realistic load you may extract half or less of their rated capacity.
This is a property of the chemistry inherently and not something a more expensive Lead Acid battery will solve, and is known as Peukert’s law[2]https://en.wikipedia.org/wiki/Peukert%27s_law.
Lithium Iron Phosphate batteries on the other hand behave much more like an ideal battery. Under a similar 50A discharge condition, a 200Ah LiFePO4 cell can be expected to lose its capacity by less than 5% –so little as to be considered inconsequential.
2. Cycle lifetime
A Lead Acid battery is capable of only about 400 cycles of rated capacity. After this point, its full storage capacity drops to 80% of what it was to begin with. It is still usable, but suffers from less capacity, greater resistance (heat) and less performance in general.
Lithium iron phosphate on the other hand, is a chemistry which lasts about 2000 cycles before reaching 80% of its original capacity — a full 5 times longer than Lead Acid cells.
3. Basic comparison
These two points considered, let’s look at the economics of these competing chemistries with some simple math.
At the time of this writing,
- A 200Ah 6 volt Lead Acid battery costs about $90. This is 1200 Watt-hours (6*200) of advertised capacity, or about $75 per kWh.
- Four 100Ah 3.2 volt LiFePO4 cells + shipping, cost $330. This is 1280 Watt-hours of advertised capacity, or about $257 per kWh.
When we factor in cycle life, the economics start to look better.
- With an expected cycle life of 400 cycles, our Lead Acid battery works out to be 18.75 cents per kWh, per cycle.
- With an expected cycle life of 2000 cycles, our LiFePO4 battery works out to be 12.85 cents per kWh, per cycle.
Now keep in mind, this is all under theoretical perfect conditions. When we actually try to draw load from our battery, the story is very different.
- With an expected cycle life of 400 cycles, under realistic load conditions of 50% capacity loss, our Lead Acid battery works out to be 37.75 cents per kWh, per cycle.
- With an expected cycle life of 2000 cycles, under realistic load conditions of 5% capacity loss, our LiFePO4 battery works out to be 13.5 cents per kWh, per cycle.
We find that the economics of LiFePO4 are to the first approximation substantially better than those of Lead Acid, without even considering that maintenance / service costs are a full 4 times less as well.
4. Further thoughts
There is another thing to consider about the Lead Acid battery chemistry which can further reduce its appeal. Notably, in storage, these batteries will permanently lose capacity at a faster rate than LiFePo4. It is very common for Lead Acid batteries to become unusable after only 5 years in storage, whereas, LiFePO4 cells manufactured a decade ago, have a capacity loss measurable in only a few percentage points.
Furthermore, when a Lead Acid battery reaches the end of its cycle life its resistance tends to be very high. Usually it is so high as to make the battery effectively unusable afterward.
A LiFePo4 battery on the other hand is still very usable at the end of its rated cycle life, and may be used for another 2000 cycles until its usable capacity has reached 60%. It may still be used for a further 2000 cycles, and if space is not of huge concern, it very well may still be worth running the battery a total of 6000 cycles down to 40% of its advertised capacity.
It is true that LiFePO4 batteries need a battery management system, but, for the purposes of energy storage, the only things which need to be considered are a circuit that will shunt the cell if its voltage exceeds 3.2V, and a circuit that disables the power draw if the cell voltage is less than 2.9. Ultimately this can amount to no more than $10 in extra circuitry per cell.
5. But what about fire?
There is a misconception that all lithium batteries are dangerous and prone to runaway combustion. This is true for LiMnCo batteries such as those used in electric cars and cell phones, but it is not very true of Li-Fe batteries. LiFePO4 batteries have a lovely property where they become less able to provide electrical current as their temperature increases. This means that they won’t easily combust unlike LiMnCo batteries, which is among one of the biggest reasons investors bet big on the new chemistry.
| ↑1 | This argument is true for LiFePO4. For LiMnCo batteries such as “Tesla” modules, this economic argument does not hold water as LiMnCo chemistry cells have a cycle life of 400 cycles or less and are not sensible for stationary energy storage use. |
|---|---|
| ↑2 | https://en.wikipedia.org/wiki/Peukert%27s_law |

Leave a Reply